Type 1 Diabetes Immunotherapy Consortium Research Update (January 2020)
3 exciting new trials for children recently diagnosed with type 1 diabetes have opened in the UK and a 4th is in set-up. These low risk immunotherapy treatments aim to slow down or halt the autoimmune destruction in Type 1 diabetes, potentially preserving some beta cell function and the body’s ability to make some of it’s own insulin.
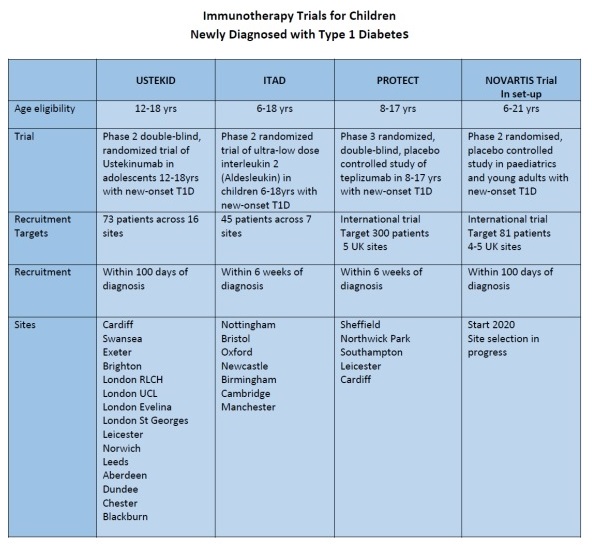
USTEKID aims to recruit 72 participants across 15 sites.
Age eligibility 12-18 years
Recruit within 100 days of diagnosis.
Sites – Cardiff, Dundee, Aberdeen, Exeter, Swansea, St Georges London, Evelina Children’s London, Royal London Children’s, Leicester, Leeds, Brighton, Norwich, Blackburn, University College London, Chester.
ITAD aims to recruit 45 participants across 7 sites.
Age eligibility 6-18 years (12-18 years initially sequential recruitment)
Recruitment within 6 weeks of diagnosis.
Sites – Bristol, Nottingham, Oxford, Newcastle, Cambridge, Manchester, Birmingham.
>>>>Details of these trials can be found on the T1D UK Immunotherapy website
PROTECT
Age eligibility 8-17 years
Recruitment within 6 weeks of diagnosis
Sites – Sheffield, Leicester, Northwick Park, Southampton, Cardiff.
>>>>Information on the PROTECT study can be found on the PROTECT website
There is a small window of opportunity for newly diagnosed patients to take part in immunotherapy trials, usually 6 weeks from diagnosis, sometimes up to 100 days, so all teams in Wales are asked to please give patients the opportunity to take part and refer them shortly after diagnosis to the T1D UK Consortium website where they can find out more about trials they may be eligible for.
If a family receive their diabetes care outside of a recruiting site they may still be able to take part. Please refer them to the T1D UK website where they can find out more, and if interested, complete the “Get Involved” enquiry form. They will be asked for brief details and referred to their nearest recruiting site. This will not involve any shift in clinical care from the local to the research team, simply a willingness on the part of the family to travel to the study site for research visits.
For further details contact: T1DUK@cardiff.ac.uk

